
What changes to the battery design result in a larger voltage or current?Ĭalculate the power output from your battery by calculating the product of its voltage and current. Use a multimeter to measure the voltage and current generated by your battery. You can compare the power qualitatively by looking at the intensity of the electrical device or quantitatively by taking measurements on a multimeter. This effectively shorts out the lower cell, which no longer contributes to the overall power output. If the foil from one cell is in contact with the foil from the cell above it, the electrons will bypass the paper towel and activated charcoal and move directly into the second piece of foil, which has a lower resistance than the charcoal layer. Press down firmly on the pile to reduce the internal resistance of the battery, but make sure that the foil pieces don’t touch each other. Clip one lead to the bottom piece of foil and place the other lead in the top charcoal pile. Stack two or three aluminum–air cells on top of each other to see if you can make a more powerful battery. To make a voltaic pile, repeat Assembly steps 1–4 to construct additional aluminum–air cells.
Salt bridge battery cathode and anode series#
The first modern electric battery was made up of a series of electrochemical cells, called a voltaic pile. This is enough power to run a small electrical device and provides a safe and easy way to make a powerful battery at home or in school. This large reaction area makes it possible for the simple aluminum–air battery to generate 1 volt (1 V) and 100 milliamps (100 mA). One gram of activated charcoal can have more internal surface area than an entire basketball court! This surface provides a large number of sites to which oxygen can bind and participate in the cathode reaction. It provides a highly porous surface that is exposed to oxygen in the air. Activated charcoal, which is mostly made of carbon, can conduct electricity and is non-reactive.
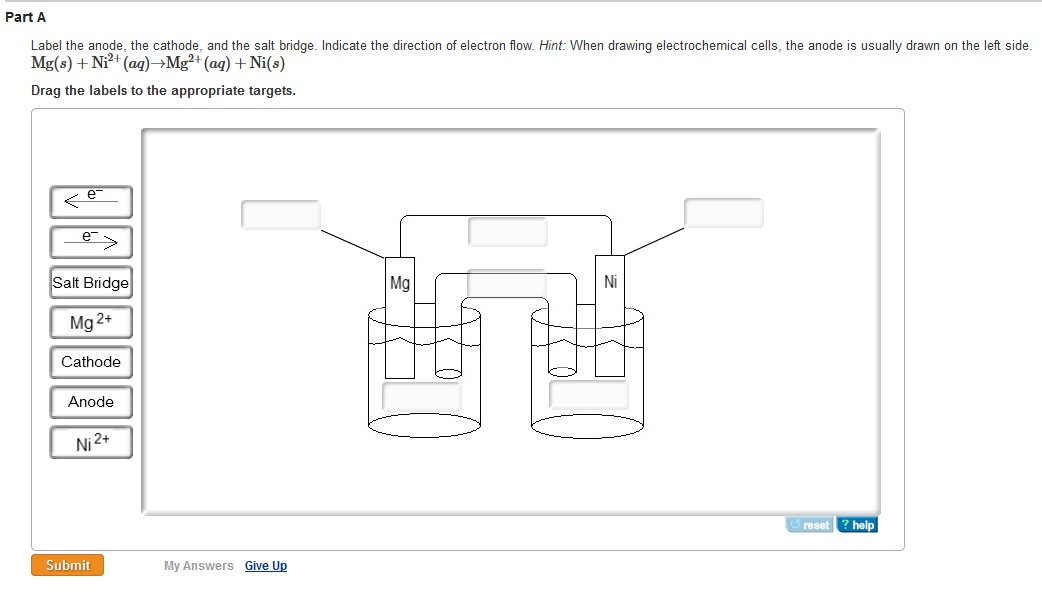
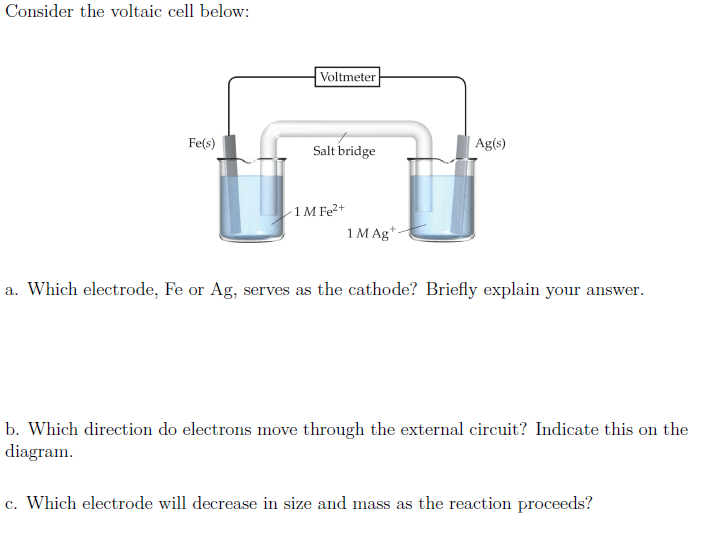
Overall: 4Al(s) + 3O 2(g) + 6H 2O(l) → 4Al(OH) 3(s)Īluminum foil provides an affordable supply of aluminum. A diagram of the battery and equations for the half and overall reactions are given below:Įquations for the half and overall reactions:Īnode: Al(s) + 3OH −(aq) → Al(OH) 3(s) + 3e −Ĭathode: O 2(g) + 2H 2O(l) + 4e − → 4OH −(aq) The movement of electrons through an external circuit generates an electric current that can be used to power simple devices. To generate electrical energy, this battery relies on oxidation of aluminum at the anode, which releases electrons, and a reduction of oxygen at the cathode, which uses electrons. In this activity, the salt provides ions that can move through the wet paper towel and transfer charge. The electrodes are connected by a solution-called an electrolyte-through which ions can move, completing an electrical circuit. They have two electrodes-called a cathode and an anode-where chemical reactions that either use or produce electrons take place. Accordingly, electrochemical cells used in batteries usually don't have a salt bridge but a semipermeable membrane instead, or a clever choice of half reactions where mixing of the electrolytes is not a problem (lead acid battery for example, where the only soluble species, sulfuric acid, is common to both half reactions).Batteries convert chemical energy into electrical energy. This tells you that the salt bridge will be depleted after a certain amount of current flows through the wire. To conclude, ions flow out of the salt bridge into the solutions A and B, but virtually no ion flows all the way through the salt bridge.
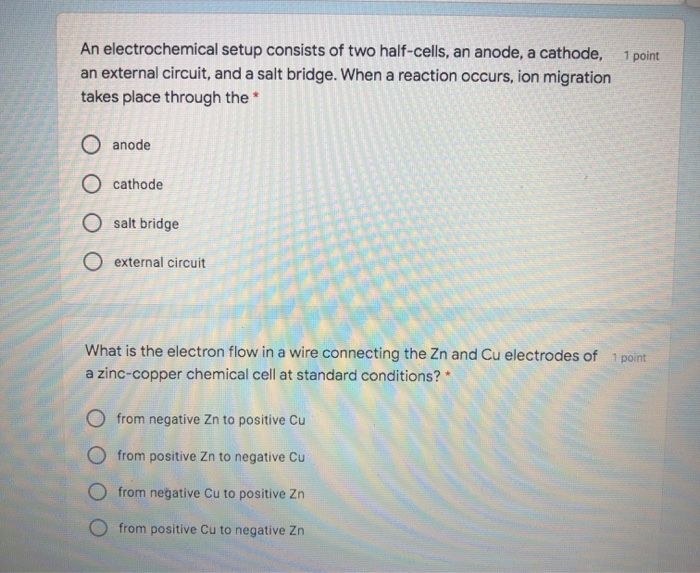
The solution in the salt bridge is not mixed, so it would take a very long time for the electrolyte in A to diffuse all the way to B. When you have a high concentration of inert ions in the salt bridge, cations in the salt bridge will flow into B, and anions in the salt bridge will flow into A. The idea of the salt bridge is to prevent electrolytes mixing while providing ion flow. According to my notes and many sources on the internet, electrons and cations both travel from the anode (A in the image) to the cathode (B in the image).


 0 kommentar(er)
0 kommentar(er)
